iHealth™ COVID-19 Antigen Rapid Test
Out of stock
iHealth COVID-19 Antigen Rapid Test is the simplest way to find out if someone has COVID-19. It only takes 4 steps and 15 minutes to complete the test. The test is also non-invasive. You won't need to collect a sample from deep in your nasal cavity to get accurate results.
New variants have shown that anyone can be vulnerable to COVID-19 even if they are vaccinated or young. With iHealth COVID-19 Antigen Rapid Test, you can test yourself after being out in public. Early and regular testing helps you better care for yourself and protects your friends, family, and community members from potential exposure.
SKU IHL00589P
iHealth COVID-19 Antigen Rapid Test is the simplest way to find out if someone has COVID-19. It only takes 4 steps and 15 minutes to complete the test. The test is also non-invasive. You won't need to collect a sample from deep in your nasal cavity to get accurate results.
New variants have shown that anyone can be vulnerable to COVID-19 even if they are vaccinated or young. With iHealth COVID-19 Antigen Rapid Test, you can test yourself after being out in public. Early and regular testing helps you better care for yourself and protects your friends, family, and community members from potential exposure.
SKU IHL00589P
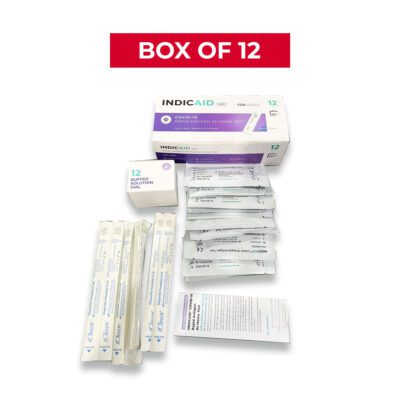
Kit Contents
- Two (2) COVID-19 test cards
- Two (2) shallow nasal swabs
- Two (2) buffer solutions tubes with caps
- Instructions for use
Performance Measures
- 94.3% Sensitivity
- 98.1% Specificity
Features
- Test for both symptomatic and asymptomatic COVID-19
- Detects both viable (live) and nonviable Covid variants
- iHealth COVID-19 Antigen Rapid Test App for easy instructions and reporting
- Two tests included for staggered testing over a 36-hour period
- Visual results available in 15 minutes
- iHealth COVID-19 Antigen Rapid Self-Test Video (CLICK HERE)
360 Health Services Acceptable Product Dating
We will ship product with expiration dating >= 30 days from order date.
Disclaimer
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
| Weight | N/A |
|---|---|
| UOM | Case/1 – 180 tests, Kit/1 – 2 tests, Pallet – 15,120 tests |
You May Also Like
INDICAID™ COVID-19 Rapid Antigen At-Home Test (Kit/12)
Out of stock